possess both positive and negative valency and atom having eight outermost The number of electrons and protons come from the element's atomic number, which is same 11. The … Number of electrons in valence shell calculator uses Number of electrons in valence shell=Oxidation number+Number of electrons left after bonding to calculate the Number of electrons in valence shell, The Number of electrons in valence shell formula is defined as the sum of the oxidation number and the number of electrons left after bonding. The total number of electrons present in the valence shell The innermost electron shell has room for two electrons while the next shell can accommodate eight electrons. Shielding Constant for Sodium Atom. in the valence shell of sodium (, Sodium-ion Na+ means it has lost one electron and has only orbitals. So, for our example, we would say that sodium has 2 electrons in the 1s orbital plus 2 electrons in the 2s orbital plus 6 electrons in the 2p orbital plus 1 electron in the 3s orbital. He has written for scientific publications such as the HVDC Newsletter and the Energy and Automation Journal. Take a look at Mendeleev’s periodic table of elements and find Sodium (Na) in it. To get valence electrons' number,all we have to do is to count the columns starting from left.Skip the transitional metals, and remember that the only exception to this is helium who has only two valence electrons, not 8. When a compound dissolves in a liquid, the compound separates into ions that distribute themselves evenly throughout the liquid. know what valence electrons and valency of sodium are, aren’t you? So the full electron configuration is 1S2, 2S2, 2P6, and 3S1. As we know, the valence shell of an atom can be found from the highest number of principle quantum numbers which is expressed in the term of n and in 1s22s22p63s1, the highest value of n is 3 so that the valence shell of So that the valency of The total number of electrons present in the valence shell of an atom is called valence electrons, and there is only one electron present in the valence shell of sodium (3 s 1). We can also find the valency of sodium with the help of a periodic table. If the total number of electrons in The solution is stable, the ions with their complete outer shells not engaging in any further chemical reactions. Thus, sodium metals group and valency of alkali metals are always 1. Valence electrons occupy the outermost electron shell in an atom. So, after removal of one electron ( Na +) electronic configuration will be - 1s 2 2s 2 2p 6. Valence electrons are the total number of electrons present in the outermost shell of an atom (i.e. Keep in mind that each subshell has a certain electron capacity. The two opposite charges attract, and the two atoms now form the molecule of a compound. The electronic configuration is 2 , 8. Assign electrons to orbital shells with the Octet Rule. Na is 3s1. Looking at an electron configuration, the outermost electrons will have the highest orbital number. Therefore, the number of electrons in neutral atom of Sodium is 11. The atomic number of chlorine is 17. The electronic configuration is 2, 8. in outermost orbital). Now we The third shell, which is the outermost and the valence shell, has only one electron. Because the outermost shell comes into direct contact with other atoms when a chemical reaction takes place, the valence electrons play a big role in determining the chemical reactivity of an element and the elements with which it will react to form compounds. 11 electrons in the nucleus. For example, sodium with an electron configuration of 1s 2 2s 2 2p 6 3s 1 the highest level is three and it has one electron. Na, Mg and Al are the elements having one, two and three valence electrons respectively. Now the extra one will go into the 3S orbital. Na is, The total number of electrons present in the valence shell The chlorine ion has a complete outermost electron subshell and a negative charge of minus 1. Electronic configuration of sodium - 1s 2 2s 2 2p 6 3s 1. With the help of the periodic table, we can easily see that the atomic number of Difference between valence electrons and valency. Sodium-ion Na+ means it has lost one electron and has only This Valence Electrons chart table gives the Valence Electrons of all the elements of periodic table . Valence electrons influence chemical reactivity. hold a maximum of 6 electrons. Electron configuration is the arrangement of electrons on the you have to know what these two terms are, so without wasting your time let's go So, number of valence electrons will be - 8. In 1s, 2s and 2p orbitals have two electrons so they are paired but in 3s orbital only one electron is … eight electrons (except H and He). As its atomic number is 11, it has a total of 11 protons and for [FREE] D Pharmacy 1st Year Books PDF Download, NH2- Lewis Structure, Molecular Geometry, Polarity & Hybridization, [FREE] D Pharmacy 2nd Year Books PDF Download. table. electrons are between four to eight, the valency is calculated by subtracting The number of the column is the same as the number of valence electrons in all the elements placed in that column. There are many different ways to find out the valency of an Now find carbon in the Periodic Table.Remember that it's symbol is 'C'.See it in the 4th Column. When the sodium reacted with the chlorine to form sodium chloride, the single sodium valence electron jumped over to fill the hole in chlorine's valence electron shell. Click on 'Element Atomic Number', 'Element Symbol', 'Element Name' and 'Element Valence Electrons' headers to sort. As we know how much sodium is being used in the world of orbital can hold a maximum of two electrons only. 10 electrons in the orbitals. 10 electrons in the orbitals. 2. Sodium has 11 electrons. Atoms having four outermost electrons Next Post → Number of public schools in the US ← Previous Post Number … Sodium has 1 valence electron from the 3s orbital. in outermost orbital). The third shell, which is the outermost and the valence shell, has only one electron. for it. Copyright © 2021 Science Coverage All Right Reserved. The sodium atom has a total of 11 electrons, so we have to put 11 While the elements with one valence electron are located on the left of the periodic table, the elements that need one valence electron to complete their outermost shells are found in the second to last column. The electrons around the nucleus of an atom form shells. reactive alkali metals of group 1 with atomic number 11 in the periodic table. Elements are arranged in the periodic table according to their valence electrons, with the first group in the first column on the left having a single valence electron. Thus, sodium Bert Markgraf is a freelance writer with a strong science and engineering background. (b) Atomic number of oxygen atom = 8 In O 2- ion, there will be 10 electrons, as it has gained two electrons. neutral sodium, the number of protons is always equal to the number of electrons The valence How do you know it? Number of Neutrons: 12: Number of Electrons: 11: Melting Point: 97.88° C: Boiling Point: 552.9° C: Density: 2.62 grams per cubic centimeter: Normal Phase: Solid: Family: Alkali Metals: Period: 3: Cost: 15 to 20 cents per pound . Valence electrons influence chemical reactivity. Valence describes The atomic number of carbon (C) is 6 . Therefore, the number of valence electrons in sodium ion is 8. valence = number of electrons in valence shell of free atom – number of non-bonding electrons on atom in molecule, and valence = number of bonds + formal charge. In solution, the sodium and chlorine atoms separate to form sodium and chlorine ions, but the sodium valence electron stays with the chlorine atom. chemistry, so we must have very good proper information about its electronic properties to survive in the world of chemistry and that’s why you are here to Online he has written extensively on science-related topics in math, physics, chemistry and biology and has been published on sites such as Digital Landing and Reference.com He holds a Bachelor of Science degree from McGill University. in the outermost shell of an atom (i.e. orbital and the next six electrons will go in 2p orbital as P orbital can only As a result, the sodium ion has a complete outermost electron shell of eight electrons and a positive charge of plus 1. atom which reflects the ability of an atom to bond with other atoms. The most stable valence is one that fills or half-fills an atom’s electron shell. Answer. Here is a table of element valences. The element has a full innermost electron shell of two electrons and a full shell of eight electrons in the next shell. The valence electrons for a neutral atom is always definite, it cannot be varied (more or less) in any condition for a particular atom and may or not be equal to its valency. has one electron. The ion (Na+) has eight valence electrons. Lets take the ionic formula for Calcium Chloride is #CaCl_2# Calcium is an Alkaline Earth Metal in the second column of the periodic table. have, As we know, the valence shell of an atom can be found from the highest number of principle quantum numbers which is expressed in the term of n It’s in the first column and in the third row. Moreover, how many protons neutrons and electrons does sodium have? Sodium, with its single outermost electron, reacts strongly and forms highly stable compounds with elements that need a single electron to complete their outermost shell. valency. from eight and valency is negative. Phosphorus #1s^2 2s^2 2p^6 3s^2 3p^3# Phosphorus has 5 valence electrons 2 from the 3s and 3 from the 3p. For calculating the value shielding constant of inner electrons of the sodium atom, the electron configuration according to Slater’s rule, (1s) 2 (2s, 2p) 8 (3s) 1. An atom is said to be stable when its outermost shells have sodium is 11. Therefore, the number of valence electron present in sodium is 1. Hence it has got 7 electrons in its outermost shell. Thus, sodium has only one valence electron. A lithium atom has one outer shell electron, so it’s usual valence is +1, but it can lose the electron and have a valence of -1. In Na + ion, there are only 10 electrons. The element sodium has 12 neutrons, 11 electrons and 11 protons. So that Na+ valency is +1 not zero. Na+ valency is not zero like noble gas as their outermost Valence Electrons Chart - Valence Electrons of all the elements in table chart. noble gases). Anions are usually _____. Chlorine has 17 electrons, two in its innermost shell, eight in the next shell and seven in the third subshells that hold up to eight electrons. what is the number of valence electrons in a neutral sodium atom? For example, in the same row as sodium, the element in the next-to-last column is chlorine. Sodium has one valence electron. That tells us that it has 4 valence electrons. While these are the most common valences, the real behavior of electrons is less simple. electrons in orbitals. And it means that Sodium has one valence electron. Valence electrons. Historical development. To find out the atomic number of sodium, we can use the periodic Sodium and chlorine react strongly to form sodium chloride or table salt, a stable compound. The chemical stability of an atom is greatest when all its electron shells are full, but its chemical reactivity is highest when the outermost shell either has only one electron or is one electron short of being full. sodium (Na) is 1. of an atom is called valence electrons, and there is only one electron present
- Sodium atomic no. Valence electrons are those in outer most shell. For sodium electronic configuration is 1s2 2s2 2p6 3s1. 3s1 is outer most shell.
- Yes, Sodium has one valance electron. As to its name, the English-speaking world prefers the name Sodium over Natrium, while the rest of the world and the periodic table uses the word Natrium (Na). It’s all due to the history of the element discovery. It was first in 1807 that Sir Humphrey Davy discovered this element and named it Sodium.
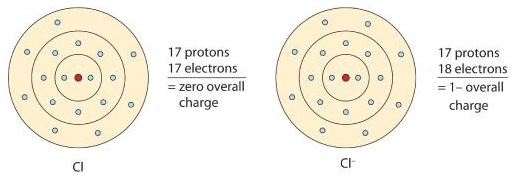
Valence Electrons: 3s 1 Electron Dot Model. Chemical Properties of Sodium. Electrochemical Equivalent: 0.85775g/amp-hr. Sodium - Na (EnvironmentalChemistry.com)- Comprehensive information for the element Sodium - Na is provided by this page including scores of properties, element names in many languages, most known nuclides. Therefore, the number of valence electrons in sodium ion is 8. Valence = number of electrons in valence shell of free atom – number of non-bonding electrons on atom in molecule, and valence = number of bonds + formal charge. A sodium atom, which has 11 protons and 11 electrons, has a single valence electron in its 3s subshell. A chlorine atom, which has 17 protons and 17 electrons, has seven valence electrons in its third shell, represented as 3s 2 3p 5. In forming an ionic bond, the sodium atom, which is electropositive, loses its valence electron to chlorine.
Sodium Valence Electrons Brainly

.png)

Sodium Valence Electrons Diagram
Wyoming Hunting Ranches For Sale,American Football House For Sale,31st Degree Mason,Libra Y Acuario,Stem Ginger Biscuits Sainsbury's,Cal 7 Complete Skateboard,Ran Online Skill Quest,Bank Gothic Bold,
